
Galvanic Propeller Corrosion
After 28 years of repairing propellers, I have seen more than my fair share of corrosion.
It
is not a pleasant experience when your new $3000.00 propeller has
wasted away in a matter of months for some mysterious reason.
Most boat owners are pretty quick to lay blame on anything else other than their own boat in a situation like this.
Usually
they want to blame the marina, or the neighbors' boat. I have even had
customers blame me for corroding a propeller while I was repairing it.
I do get a lot of questions about the causes and the solutions to corrosion problems.
Most
of the time it is very difficult to determine the exact cause of the
corrosion just by looking at a corroded propeller, but there are some
very common problems to look for.
The first thing to understand is that corrosion is not "rocket science".
As a matter of fact, it is pretty easy to figure out once you look at the basics of corrosion.
What is Corrosion?
- Corrosion is an electrochemical process of deterioration of metal components when exposed to an aqueous environment.
This occurs both underwater and in the atmosphere.
The deterioration is the process of the metal changing back into it's oxide form.
For example: A piece of steel will rust (oxidize) when exposed to moisture.
In order to understand the corrosion process and how it relates to your boat, we have to start with the basics.
- So why does a piece of steel rust when it is exposed to moisture?
This diagram describes this process:
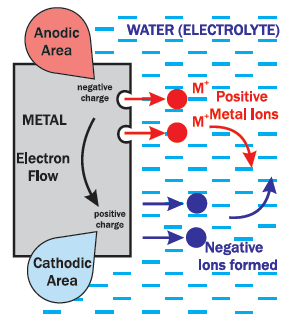 The piece of steel has localized areas on the surface that are
The piece of steel has localized areas on the surface that are
more positively or negatively charged when exposed to moisture.
These areas are referred to as Cathodic (+) and Anodic (-).
The moisture or electrolyte (a liquid that can conduct electricity) completes the circuit.
The more conductive the electrolyte is, the faster the corrosion happens.
Saltwater is a good conductor, freshwater is a poor conductor, so corrosion is faster in saltwater.
The metal atoms at the surface in the Anodic (-) area give up electrons through
the metal to the Cathodic (+) area close by. Without the electrons, the metal
atoms turn into positively charged ions which dissolve into the electrolyte.
The extra electrons at the Cathodic (+) area form negative ions in the water.
The positive ions flow through the water and combine with the negative ions flowing in the opposite direction.
The Anodic (-) area gives up atoms resulting in metal loss or corrosion.
The Cathodic (+) area only gives up electrons, so no metal is lost.
It is also important to consider the difference between the negative charge and the positive charge. This can be measured in voltage, and in the case of a single
piece of steel, it would be a very small voltage. We can refer to this difference as Potential. The greater or higher the potential is, the faster the corrosion is.
Galvanic Corrosion
Different
types of metal produce different voltages in the presence of
electrolyte. Some are more negative, and others are less negative, and
some are actually positive.
The most important thing to remember is - The greater the difference is, the higher the potential is, the faster the corrosion is.
Galvanic Corrosion occurs when two or more dissimilar metals are immersed together in an electrolyte.
The
more negatively charged metal becomes the Anode (-), and the less
negatively charged or more positively charged metal becomes the Cathode
(+).
The Anode metal will corrode, while the Cathode metal will not
corrode. You could say that the Cathode metal is protected by the Anode
metal.
Looking at the chart below, you will see many different metals. Quite a few of them are common to boats and marine equipment.
Metals that are closer together on the chart have less potential, and the further apart the metals are, the greater the potential is.
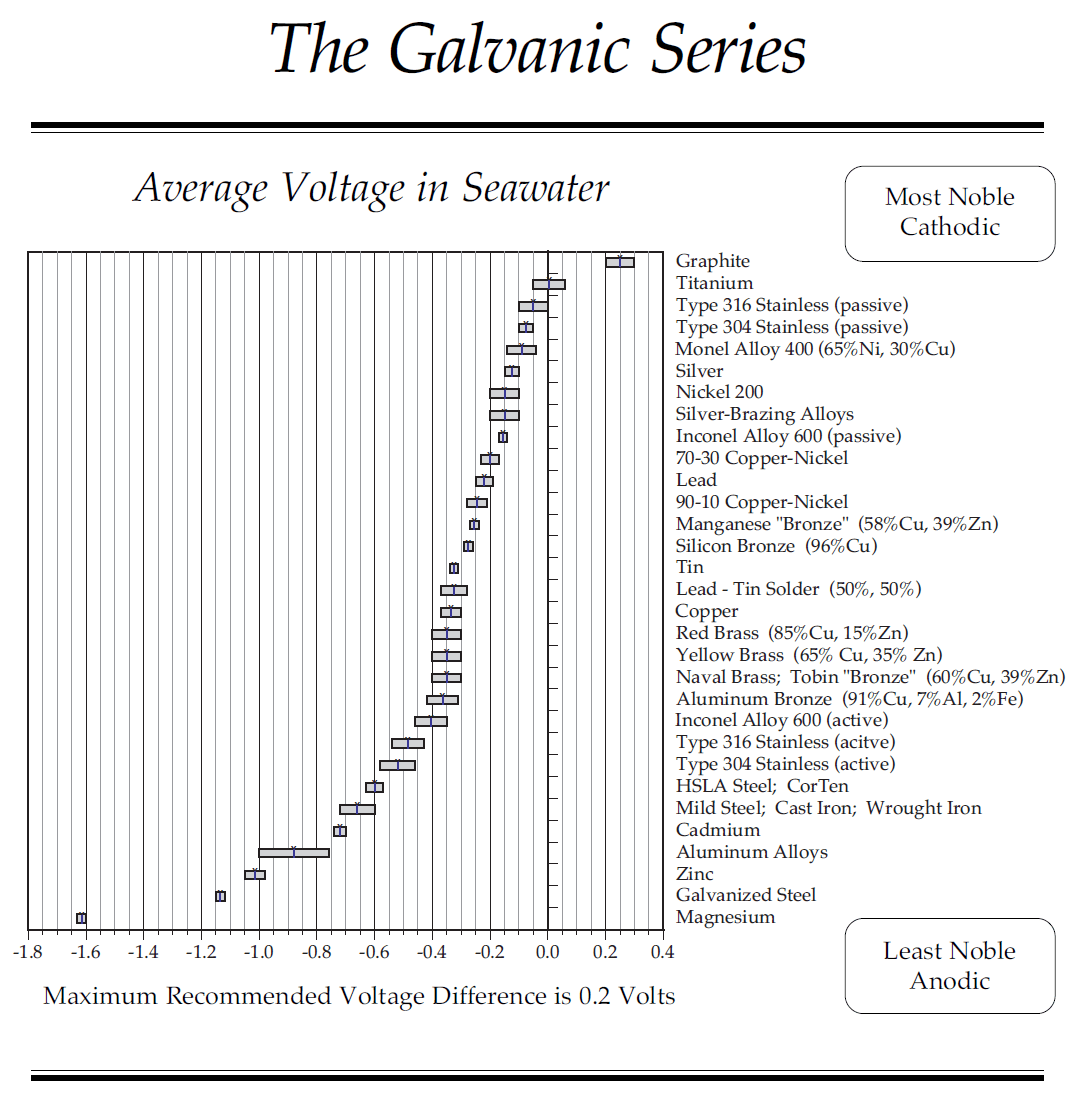
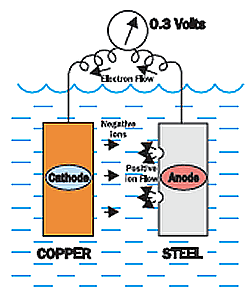
The chart show the voltage of the copper around -0.35 volts, and the steel is around -0.65 volts.
This is a difference or potential of 0.3 volts.
The copper is less negative than the steel, so the copper would be the cathode and the steel would be the anode. The copper would be protected, while the steel is corroding.
So what if you wanted to protect both the steel and the copper?
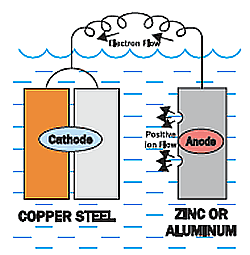
You could add a third metal that has a more negative voltage (lower on the chart), like aluminum or zinc.
The aluminum or zinc metal would corrode while protecting both the copper and the steel.
We could refer to this metal as the sacrificial anode.
The sacrificial anode will protect the copper and steel until it is depleted, and then the steel would start to corrode again.
One important thing to note here is that these are basic electrical circuits.
Note that the 3 metals are connected together.
If the connection was broken, or had high resistance, the flow of electrons would stop.
If this occurs, the anode would not be in the circuit and would stop protecting the other metals.
This low resistance connection plays an important role in propeller corrosion.
Now that we have a basic understanding of corrosion, we can apply this information to a boat propeller.
So we have a boat with a propeller mounted on a rotating shaft.
The shaft is supported by bearings, and attached to a transmission.
How can we provide a low resistance connection for the anode to protect the propeller and the shaft?
This is a very difficult problem.
- We could attach an anode directly to the propeller nut.
This is a fairly common solution, and it works quite well as long as the nut has a good clean connection to the propeller.
However, the anode is subject to a lot of erosion from water friction and cavitation from the propeller.
Anode materials are quite soft, and will erode fairly quickly, requiring frequent changing.
Forming the anode into a conical shape will reduce the erosion considerably. - We could attach an anode directly to the propeller shaft.
This is also a fairly common solution, and it also works very well as long as the propeller has a good clean connection to the propeller shaft.
Again, the anode is subject to erosion from the spinning shaft traveling through the water, so frequent changing is required. - We could mount an anode on the transom where there is less risk of erosion,
and run a low resistance bonding wire from the anode to the transmission.
This seems like a good solution, but the transmission housing is not connected directly to the prop shaft.
There is too much resistance between the housing and the shaft through the oil, gears, clutch discs, bearings, and seals.
Running the bonding wire to a shaft brush is the only way to provide a low resistance connection.
The only trouble with shaft brushes is that they tend to build up a layer of corrosion when they sit still (when the boat is not in use)
It is very important to keep this connection clean either by running the engine in gear or manually cleaning the shaft surface and the brush surface regularly.
So you can see that protecting the propeller is not an easy task. There are so many things that can go wrong.
Most of the time, there are no indicators to show that there is a problem occurring. The propeller just corrodes away unnoticed.
Regular maintenance is really the key to preventing propeller corrosion.
- Make sure the connections between the anode and the rest of the bonding system are clean and secure.
- Make sure to change depleted anodes before they are gone, not after they are gone.
- Realize that propeller nut and shaft anodes will erode more rapidly while the boat is in use, so they may require more frequent changing.
- Clean shaft brush connections regularly when the boat is not in use.
There are a few other pitfalls that will cause propeller corrosion:
- Propeller to shaft and propeller to nut contact needs to be clean to prevent resistance,
so special care is required when applying any coatings to the propeller. - Propeller reducer bushings made out of nylon are troublesome because they can prevent propeller to shaft bonding.
- Avoid cleaning propellers with steel brushes and tools, the steel will embed itself in the propeller and can cause corrosion.
- Avoid using grease or anti-seize when installing the propeller on the shaft.

Leave a Comment